World Society of Pediatric Ophthalmology and Strabismus
A Comprehensive Update on
Myopia Management
Independent medical education support provided by
EssilorLuxottica (Gold), Hoya (Silver) and Cooper Vision (Bronze)
Table of Contents

- Defining the Problem: Changing Views on the Impact of Myopia - Ramesh Kekunnaya
- Reviewing the Available Tools to Manage Myopia Today
- Overnight Orthokeratology: a Closer Look at Corneal Reshaping - Mark A. Bullimore
- New Daily-Wear Contact Lenses Designed for Myopia Control - Erin S. Tomiyama
- Preventing Axial Length Elongation with a Pair of Spectacles: PALs, Bifocal Approaches, and Peripheral Diffusion Technology - Ian Flitcroft
- Preventing Axial Length Elongation with a Pair of Spectacles: DIMS and HAL Technology - Carly Lam
- The Science Behind Pharmacological Modulation for Myopia Control - Seo Wei Leo
- Best Practices for Patient Education - Célia Nakanami

Defining the Problem: Changing Views on the Impact of Myopia


Ramesh Kekunnaya,
MD, FRCS
Director of Child Sight Institute and
Center for Technology Innovation at L V
Prasad Eye Institute, Hyderabad, India.
rameshak@lvpei.org
Introduction
* The WSPOS Myopia Survey was completed in November 2022 as part of the Independent Medical Education (IME) Programme on Comprehensive Update on Myopia Management. A total of 326 respondents from 64 countries participated in the survey, which included 11 questions.
Prevalence
Pathology
The impact of myopia on patient’s quality of life
Myopia Progression

Myopia is a global epidemic! It poses an economic burden and results in diminished quality of life.

Children are developing myopia at an earlier age!
Diagnosis
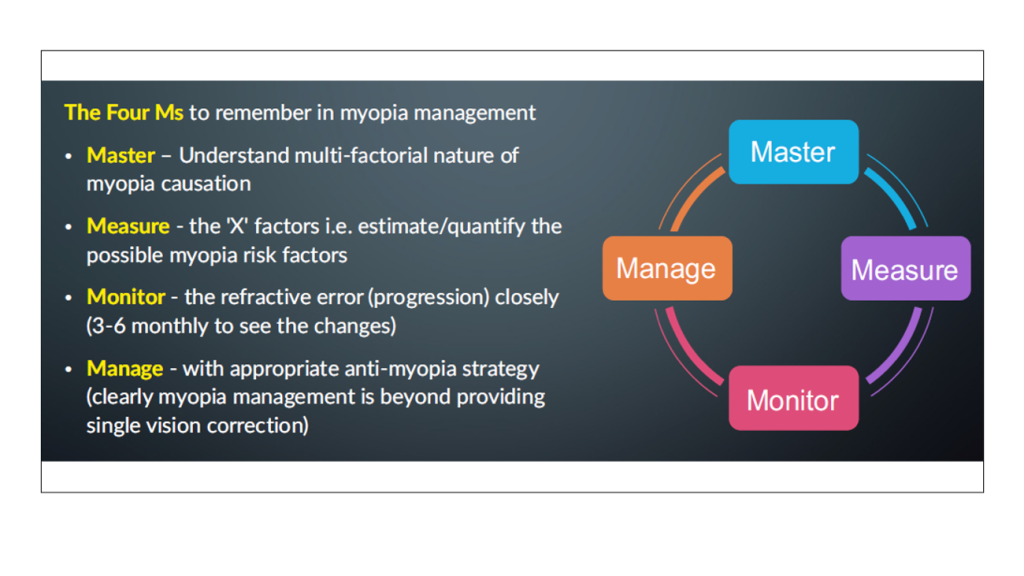
Until this epidemic is controlled, it is important to remember the so-called myopia mantra: Master, Manage, Measure, and Monitor [Figure 2].
Click to expand
Figure 2. The Myopia Mantra. [Courtesy of Pavan Verkicharla]
² Sankaridurg, P. et al. IMI impact of myopia. Investigative Ophthalmology and Visual Science 62(5):2 (2021).
³ Meng, W., Butterworth, J., Malecaze, F. & Calvas, P. Axial length of myopia: A review of current research. Ophthalmologica vol. 225(3): 127–34 (2011).
⁴ Mutti, D. O. et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci 48(6): 2510–9 (2007).
⁵ Williams, K. & Hammond, C. High myopia and its risks. Community Eye Health Journal 32(105): 5–6 (2019).
⁶ Tideman, J. W. L. et al. Association of axial length with risk of uncorrectable visual impairment for europeans with myopia. JAMA Ophthalmol 134(12): 1355–63 (2016).

Reviewing the Available Tools to Manage Myopia Today
Overnight Orthokeratology: a Closer Look at Corneal Reshaping


Mark A. Bullimore,
OD, PhD
Independent Regulatory Consultant and
Adjunct Professor at University of Houston,
College of Optometry, Houston, Texas, USA.
bullers2020@gmail.com
Orthokeratology
Axial Elongation
The first publication of the effect of orthokeratology on slowing myopia was published 18 years ago², showing that treatment with orthokeratology could reduce about 0.25mm in axial length elongation. This effect was consistent in subsequent studies and irrespective of lens designs, manufactures, and geographic location³ [Figure 1]. Also consistent across myopia control treatments, including orthokeratology, was the more dramatic slowing of axial elongation during the first year¹ [Figure 2].

When it comes to evaluating myopia control methods, axial length is becoming the method of choice for a number of reasons.
Click to expand
Benefits and Risks

The benefits of myopia control with contact lenses and other modalities far outweigh the risks.
Unanswered questions
There are, still, many unanswered questions to be addressed. Achieving a better understanding of the efficacy of orthokeratology on different levels of myopia is crucial. Evidence suggests that orthokeratology is more effective at higher levels of myopia² and it can be partially corrected at higher levels of myopia¹⁰. Less clear is how the manipulation of central and peripheral zones alters the efficacy of orthokeratology in slowing axial elongation. One study suggests that a smaller optical zone is more effective in slowing axial elongation¹¹. Under investigation is also the use of combined therapies. Several studies considered the combination of atropine with optical modalities. However, atropine seems to have a modest additive effect that is more pronounced at lower levels of myopia than at higher levels of myopia¹².
² Cho, P., Cheung, S. W. & Edwards, M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: A pilot study on refractive changes and myopic control. Curr Eye Res 30(1): 71–80 (2005).
³ Wen, D. et al. Efficacy and acceptability of orthokeratology for slowing myopic progression in children: A systematic review and meta-Analysis. Journal of Ophthalmology: 360806 (2015).
⁴ Hiraoka, T., Kakita, T., Okamoto, F., Takahashi, H. & Oshika, T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: A 5-year follow-up study. Invest Ophthalmol Vis Sci 53(7): 3913–9 (2012).
⁵ Santodomingo-Rubido, J., Villa-Collar, C., Gilmartin, B., Gutiérrez-Ortega, R. & Sugimoto, K. Long-term Efficacy of Orthokeratology Contact Lens Wear in Controlling the Progression of Childhood Myopia. Curr Eye Res 42(5): 713–720 (2017).
⁶ Bullimore, M. A., Sinnott, L. T. & Jones-Jordan, L. A. The risk of microbial keratitis with overnight corneal reshaping lenses. Optometry and Vision Science 90(9): 937–44 (2013).
⁷ Bullimore, M. A. et al. Pediatric Microbial Keratitis with Overnight Orthokeratology in Russia. Eye Contact Lens 47(7): 420–25 (2021).
⁸ Bullimore, M. A. et al. The Risks and Benefits of Myopia Control. Ophthalmology 128(11): 1561–1579 (2021).
⁹ Vincent, S. J. et al. CLEAR – Orthokeratology. Contact Lens and Anterior Eye 44(2): 240–69 (2021).
¹⁰ Charm, J. & Cho, P. High myopia-partial reduction ortho-k: A 2-year randomized study. Optometry and Vision Science 90(6): 530–9 (2013).
¹¹ Guo, B., Cheung, S. W., Kojima, R. & Cho, P. One-year results of the Variation of Orthokeratology Lens Treatment Zone (VOLTZ) Study: a prospective randomised clinical trial. Ophthalmic and Physiological Optics 41(4): 702–714 (2021).
¹² Kinoshita, N. et al. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep 10(1):12750 (2020).
Dr. Mark Bullimore is a consultant for Alcon, CooperVision, EssilorLuxottica, Euclid Systems, Eyenovia, Genentech, Johnson & Johnson Vision, Lentechs, Novartis, Oculus, Paragon Vision Sciences, and Vyluma.

New Daily-Wear Contact Lenses Designed for Myopia Control


Erin S. Tomiyama
OD, PhD
Assistant Professor of Optometry at Marshall
B. Ketchum University, California, USA.
etomiyama@ketchum.edu
When to start and stop myopia management with SCL?
Myopia can progress until early adulthood¹, so these children might need SCLs for many years. According to the COMET study, 50% of children’s myopia tends to stabilize by age 15 and 75% by age 18². This means, however, that 50% of children continue to progress after age 15 and 25% after age 18. At that time, daily activities like driving and sports that require sharper acuity or better visual performance might prompt teenagers to stop myopia management SCL. In these cases, it is still important to continue monitoring the progression and look for any rebound effect. Even though, there is no evidence of a rebound effect in children up to 7 years after cessation of SCLs ³,⁴. Treatment can always be restarted if needed.

Myopia management with soft contact lenses should start as soon as the child is able to handle contact lenses wear.
Deciding between Orthokeratology versus SCL
Deciding which SCL
Options in the US
Other multifocal lenses are available for myopia control, but these are considered off-label. Fortunately, children adapt well to a variety of modalities, lens materials, and designs. The center-distance CooperVision Biofinity and Proclear lenses allow distance correction in the 1.5mm central optic zone and variable add powers into the periphery, up to +4D with Proclear and up to +2.5D with Biofinity [Figure 1B]. Both have toric options. Another off-label option is the extended depth of focus (EDOF) multifocal, VTI NaturalVue [Figure 1C]. There is an absence of a spherical central optic zone and a rapid, continuous increase in add power from the center to the periphery, creating a “virtual pinhole” that allows an extended depth of focus. They have a universal add power that is effective up to +3D. Noteworthy, there is currently limited myopia management data with these lenses.
Click to expand
Figure 1. SCLs available in the US A) Concentric ring multifocals design in MiSight lenses. B) Center
distance
(D-lens) design in Biofinity/Proclear lenses. C) EDOF multifocal VTI. [Courtesy of Erin Tomiyama]
Options outside the US
Click to expand
Figure 2. SCLs available outside the US A) SwissLens Relax B) Esencia C) Mark’ennovy
Mylo lens. [Courtesy of Erin Tomiyama]
Conclusion

I usually go over all of the options, but I do make a clear recommendation because that’s why your patient is coming to see you and wanting you to take care of their child’s vision.
² Hardy, R. et al. Myopia stabilization and associated factors among participants in the correction of myopia evaluation trial (COMET). Invest Ophthalmol Vis Sci 54(13): 7871–84 (2013).
³ Chamberlain, P., Arumugam, B. et al. Myopia Progression on Cessation of Dual-Focus Contact Lens Wear: MiSight 1 day 7-Year Findings. Optom. Vis. Sci. (E-abstract) 98: 210049 (2021).
⁴ Hammond, D., Arumugam, B. et al. Myopia Control Treatment Gains are Retained after Termination of Dual-focus Contact Lens Wear with No Evidence of a Rebound Effect. Optom. Vis. Sci. (E-abstract) 98: 215130 (2021).
⁵ Chalmers, R. L. et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci 52(9): 6690–6 (2011).
⁶ Chamberlain, P. et al. A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optometry and Vision Science 96(8) 556–67 (2019).
⁷ Chamberlain, P. et al. Long-term Effect of Dual-focus Contact Lenses on Myopia Progression in Children: A 6-year Multicenter Clinical Trial. Optometry and Vision Science 99(3): 204–12 (2022).
Dr. Erin Tomiyama is a consultant for CooperVision Specialty EyeCare and Vyluma.

Preventing Axial Length Elongation with a Pair of Spectacles: PALs, Bifocal Approaches, and Peripheral Diffusion Technology


Ian Flitcroft
MA D.Phil, FRCOphth
Adjunct Professor of Vision Science at
Technological University Dublin and
Associate Clinical Professor of
Ophthalmology at University College Dublin,
Dublin, Ireland.
ian@flitcroft.com
Where do spectacles fit in?
Executive Bifocals and PALs Spectacles
The Correction of Myopia Evaluation Trial (COMET) was particularly important in showing that optics can indeed control eye growth and confirmed the role of defocus in the progression of myopia¹. The only caveat is that while the effect was statistically significant, it was not clinically impactful. The PALs intervention had a relatively small magnitude of effect over 3 years and most of it during the first year. There was, however, a sub-group of kids with esophoria/large accommodative lags who had a greater benefit over the three years, but less than what is achieved with other technologies. Results from studies with bifocals have been more inconsistent. Some studies had convincingly shown no effect on axial length while others had shown an apparent effect²,³. It is, therefore, no surprise that bifocals have come in and out of fashion over 30 or more years.
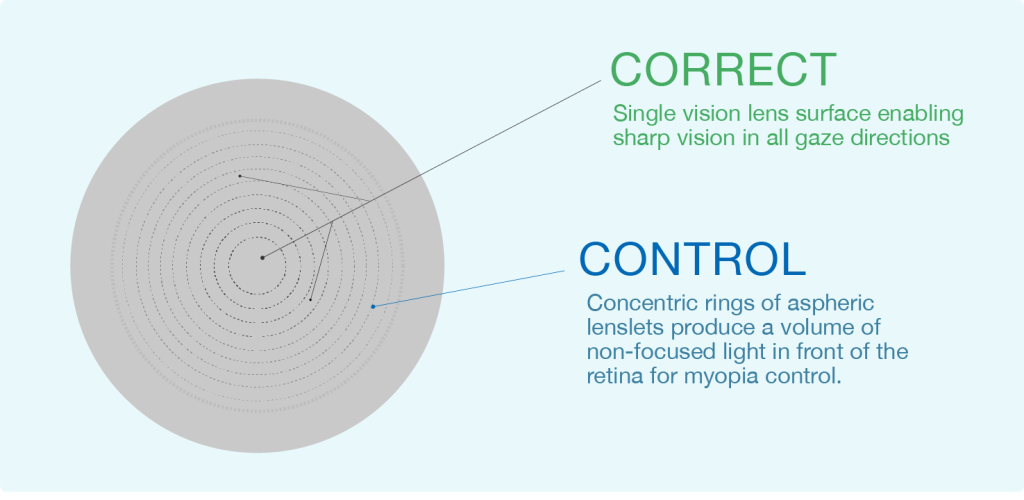
Highly Aspheric Lenslet (HAL)
Click to expand
Figure 1. Concept of Highly Aspheric Lenslet (HAL) Technology.
How should we judge success?

Correct and control, this is the concept that I use when I explain this to parents.
Click to expand
Figure 2. Rate of myopia progression and axial elongation with HAL, SAL, and SVL over a period of 2 years. Adapted from Figure 2 in ⁴.
Spectacles are an integral part of any myopia control strategy
Modern myopia control glasses are an effective and ideal starting point for many children. Latest technologies have been pioneered in Asia and more studies in European children are needed to look at efficacy, acceptability, use patterns, and impact on life quality.

A very exciting era to be heading into!
² Grosvenor, T., Perrigin, D. M., Perrigin, J. & Maslovitz, B. Houston myopia control study: A randomized clinical trial. part II. final report by the patient care team. Optometry and Vision Science 64(7): 482–98 (1987).
³ Cheng, D., Woo, G. C., Drobe, B. & Schmid, K. L. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: Three-year results of a randomized clinical trial. JAMA Ophthalmol 132(3): 258–64 (2014).
⁴ Bao, J. et al. Spectacle Lenses With Aspherical Lenslets for Myopia Control vs Single-Vision Spectacle Lenses: A Randomized Clinical Trial. JAMA Ophthalmol 140(5): 472–478 (2022).
⁵ Drobe, B. et al. Influence of wearing time on myopia control efficacy of spectacle lenses with aspherical lenslets. Invest. Ophthalmol. Vis. Sci. 63(7): 4324-A0029 (2022).
⁶ Weng, R. et al. Progression of myopia with novel myopia control spectacle lenses. Invest. Ophthalmol. Vis. Sci. 63(7): 252-A0106 (2022).
⁷ Gao, Y., Lim, E. W., Yang, A., Drobe, B. & Bullimore, M. A. The impact of spectacle lenses for myopia control on visual functions. Ophthalmic Physiol Opt 41(6): 1320–1331 (2021).
⁸ Huang, Y. et al. Effect of spectacle lenses with aspherical lenslets on choroidal thickness in myopic children: a 2-year randomised clinical trial. British Journal of Ophthalmology: bjophthalmol-2022-321815 (2022).
Dr. Ian Flitcroft is consultant ophthalmologist at Children’s University Hospital, Temple Street and a consultant/advisory board
member for Dopavision, Essilor, Johnson & Johnson Vision, and Thea. Dr. Flitcroft is also co-founder of Ocumetra Ltd, where
he is the Medical Director and Head of Research and Development. He’s received research funding from Health Research
Board (Ireland), Vyluma, and CooperVision; and has two patents pending (one in myopia management data analytics and one
in biomonitoring for low dose atropine treatment in myopia).

Preventing Axial Length Elongation with a Pair of Spectacles: DIMS and HAL Technology


Carly Lam
PhD, Professor
Professor at Center for Myopia
Research, School of Optometry at
the Hong Kong Polytechnic
University and Center for Eye and
Vision Research (CEVR), Hong Kong.
carly.lam@polyu.edu.hk
Peripheral defocus
Click to expand
Figure 1. A) Uncorrected myopia with peripheral hyperopic and myopic defocus. Adapted from ¹. B) Myopia correction
using traditional contact lens. Adapted from ². C) Optimal correction in myopia. Adapted from ³.
Reduction of peripheral hyperopic defocus
Contrast reduction between adjacent photoreceptors
Defocus Incorporated Multiple Segments (DIMS)
Conclusion

Preventing Axial Elongation with a pair of spectacles can be done!

The ultimate goal of controlling myopia progression is to eliminate ocular-related pathologies.
² Lin, Z. et al. Peripheral defocus with single-vision spectacle lenses in myopic children. Optometry and Vision Science 87(1): 4–9 (2010).
³ Smith III, E. L. The Charles F. Prentice Award Lecture 2010. A case for peripheral optical treatment strategies for myopia.
Optom Vis Sci 88(9): 1029–44 (2012).
⁴ Sankaridurg, P. et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optometry and Vision Science 87(9): 631–41 (2010).
⁵ Kanda, H. et al. Effect of spectacle lenses designed to reduce relative peripheral hyperopia on myopia progression in Japanese children: a 2-year multicenter randomized controlled trial. Jpn J Ophthalmol 62(5): 537–43 (2018).
⁶ Rappon, J. et al. Control of myopia using diffusion optics spectacle lenses: 12-month results of a randomised controlled, efficacy and safety study (CYPRESS). British Journal of Ophthalmology: bjophthalmol-2021-321005 (2022).
⁷ Rappon, J., Neitz, J., Neitz, M., Chung, C. & Chalberg, T. W. Two-year effectiveness of a novel myopia management spectacle lens with full-time wearers. Invest Ophthalmol Vis Sci 63(7): 408 (2022).
⁸ Lam, C. S. Y. et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomised clinical trial. British Journal of Ophthalmology 104(3): 363–368 (2020).
⁹ Lam, C. S. Y. et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: results of a 3-year follow-up study. British Journal of Ophthalmology 106(8): 1110-14 (2022).
¹⁰ Lam, C. S. Y. et al. Long-term myopia control effect and safety in children wearing DIMS spectacle lenses for 6 years. Sci Rep 13(1): 5475 (2023).
¹¹ Kaymak, H. et al. Myopia treatment and prophylaxis with defocus incorporated multiple segments spectacle lenses. Ophthalmologe 118(12): 1280–86 (2021).
Dr. Carly Lam is involved in collaborative research sponsored by HOYA Lens Thailand Ltd, subsidiary of HOYA Corporation
(Tokyo, Japan), with whom she co-developed the DIMS patent.

The Science Behind Pharmacological Modulation for Myopia Control

Seo Wei Leo
MBBS, FRCSEd
Medical Director at Dr Leo Adult
& Paediatric Eye Specialists Pte
Ltd, Mount Elizabeth Medical
Center, Singapore.
drleosw@gmail.com
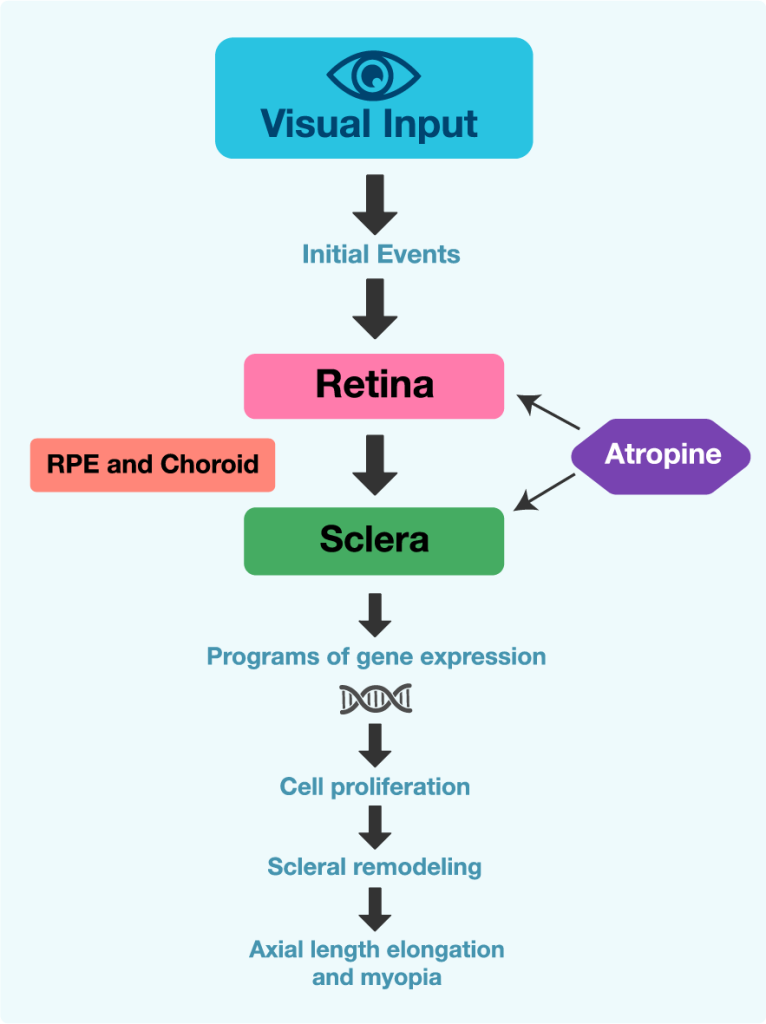
How does atropine work in myopia?
Lower or higher dosage?
ATOM1 and ATOM2 studies
The ATOM2 study [Figure 2] further investigated the effect of lower atropine doses (0.5%, 0.1%, and 0.01%) on myopia progression and AL⁷. The effect was found dose dependent but with comparable efficacy. Atropine 0.01% had a negligible effect on accommodation and pupil size, no effect on near vision acuity, minimal side effects (allergic conjunctivitis and dermatitis), and less rebound⁸. Among the dosages tested, atropine 0.01% was considered the most effective in slowing myopia progression over a period of 5 years⁹.
Click to expand
Figure 2. Summary findings from the ATOM1 and ATOM2 studies.
Adapted from Figure 6 in ⁹.
LAMP study
Although there were subtle differences between the ATOM and LAMP studies, namely LAMP participants were younger, 0.01% atropine in the ATOM2 study showed similar anti-myopia effect as 0.05% atropine and stronger effect than 0.01% atropine in the LAMP study. In the LAMP study, 0.05% atropine was found as the optimal concentration over a period of 3 years¹². Stopping the treatment at an older age and lower concentration were also associated to smaller rebound.
WA-ATOM study
Meta-analyses studies
When to start and stop atropine?
As in all eyedrops treatments, especially in paediatric patients, administration can be challenging. Children might be resistant to the administration of the eyedrops and parents might be reluctant to administer them. Developing administration methods and providing guidance on their administration are additional challenges. Currently in the pipeline is a microdose formulation of atropine (MicroPine by Eyenovia), that allows children to self-administer. MicroPine reduces dose-related side effects and presents Bluetooth capability to ensure patient adherence. A clinical trial is in progress.
Conclusion

Combination therapy is the most exciting phase in myopia control!
² Yen, M. Y., Liu, J. H., Kao, S. C. & Shiao, C. H. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol 21(5): 180–2, 187 (1989).
³ Shih, Y. F. et al. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand 79(3): 233–36 (2001).
⁴ Shih, Y. F. et al. Effects of different concentrations of atropine on controlling myopia in myopic children. Journal of Ocular Pharmacology and Therapeutics 15(1): 85–90 (1999).
⁵ McBrien, N. A., Moghaddam, H. O. & Reeder, A. P. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci 34(1): 205–15 (1993).
⁶ Chua, W. H. et al. Atropine for the Treatment of Childhood Myopia. Ophthalmology 113(12): 2285–91 (2006).
⁷ Chia, A. et al. Atropine for the treatment of childhood Myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 119(2): 347–54 (2012).
⁸ Chia, A. et al. Atropine for the treatment of childhood myopia: Changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 157(2): 451–7 (2014).
⁹ Chia, A., Lu, Q. S. & Tan, D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2 Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology 123(2): 391–9 (2016).
¹⁰ Loh, K., Lu, Q., Tan, D. & Chia, A. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol 159(5): 945–9 (2015). ¹¹ Yam, J. C. et al. Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology 127(7): 910–19 (2020).
¹² Yam, J. C. et al. Three-Year Clinical Trial of Low-Concentration Atropine for Myopia Progression (LAMP) Study: Continued Versus Washout: Phase 3 Report. Ophthalmology 129(3): 308–21 (2022).
¹³ Lee, S. S. Y. et al. Low-concentration atropine eyedrops for myopia control in a multi-racial cohort of Australian children: A randomised clinical trial. Clin Exp Ophthalmol 50(9): 1001–12 (2022).
¹⁴ Tsai, H. R., Chen, T. L., Wang, J. H., Huang, H. K. & Chiu, C. J. Is 0.01% atropine an effective and safe treatment for myopic children? a systemic review and meta-analysis. J Clin Med 10(17): 3766 (2021).

Best Practices for Patient Education

Célia Nakanami
MD, PhD
Chair of Pediatric Ophthalmology
and Low Vision at Federal University
of Sao Paulo (UNIFESP),
Sao Paulo, Brazil.
ce.nakanami@gmail.com
How to communicate with parents and patients?
Eye care practitioners should communicate clearly with the parents to ensure they understand that their child has myopia, which tends to progress and needs to be properly addressed. Providing relevant evidence is important, but parents should not be overloaded with too much information. Showing illustrations or using didactic eye models might be helpful during the initial conversation. On the other hand, scientific terms or words that might scare the parents, or the child must be avoided. Guidelines from organizations like the International Myopia Institute (IMI)³ and recommendations from eye care societies are accessible and should be considered.

Parent and patient education is crucial in myopia management.
What should be included in a discussion about myopia management?
Parents need to understand that the treatment should start as early as possible, and that several safe and effective interventions to control myopia progression are currently available⁷. Treatment plans should be provided and properly explained in terms of their efficacy, benefits, and risks. Several interventions are still off-label in most countries so parents should be informed. Parents must also understand that these are long-term treatments (≥ 2 years) possibly until eye growth stabilizes (≥ 15 year-old) and that not all patients respond to treatment. Over the years, the initial treatment can also change or be combined with other interventions. Setting realistic expectations and goals and establishing healthy lifestyle habits are extremely important even before starting the treatment.
During the diagnosis it is important to acquire relevant information such as age of onset, refractive status of the parents, and visual environment (education intensity and time outdoors) and perform baseline exams to assess refractive error and binocular visual function [Figure 1].
Children Compliance
How to convince the child to be compliant?
Modification of environmental factors
Conclusion
Click to expand
Figure 2. Goals of myopia management.
² Yang, A., Pang, B. Y., Vasudevan, P. & Drobe, B. Eye Care Practitioners Are Key Influencer for the Use of Myopia Control Intervention. Front Public Health 10: 854654 (2022).
³ Gifford, K. L. et al. IMI – Clinical management guidelines report. Invest Ophthalmol Vis Sci 60(3): M184–M203 (2019).
⁴ Limwattanayingyong, J., Amornpetchsathaporn, A., Chainakul, M., Grzybowski, A. & Ruamviboonsuk, P. The Association Between Environmental and Social Factors and Myopia: A Review of Evidence From COVID-19 Pandemic. Front Public Health 10: 918182 (2022).
⁵ Wang, J. et al. Progression of Myopia in School-Aged Children after COVID-19 Home Confinement. JAMA Ophthalmol 139(3): 293–300 (2021).
⁶ Morgan, I. G. et al. IMI risk factors for myopia. Invest Ophthalmol Vis Sci 62(5): 3 (2021).
⁷ Chia, A. & Tay, S. A. Clinical management and control of myopia in children. In: Ang, M. & Wong, T. (eds.) Updates on Myopia, Springer (2020).
⁸ Winnick, S., Lucas, D. O., Hartman, A. L. & Toll, D. How do you improve compliance? Pediatrics 115(6): e718-24 (2005).
⁹ Eppenberger, L. S. & Sturm, V. The role of time exposed to outdoor light for myopia prevalence and progression: A literature review. Clinical Ophthalmology 14: 1875–90 (2020).
Dr. Célia Nakanami is council member of the Brazilian Society of Pediatric Ophthalmology (SBOP) and consultant of Essilor
Luxottica.